Estera Lesnik
Student Profile
Name of student:
Estera Lesnik
Summary Institutes/universities attend and attending:
Master student at University of Bradford
Your current job/employment/research (max 100 words):
I am a student at Bradford University doing MSc in Chemistry. I have finished BSc and currently I am doing my Masters project at UniCRE as part of Industrial Experience. The focus of my research project is on Cannizzaro reaction. It is an organic reaction of furfural and solid base to produce furoic acid and alcohol. This reaction proceeds in the presence of hydrotalcite. There is assumption that furoic acid produced in this reaction blocks active sites of hydrotalicte decreasing furfural conversion.
Research carried out at VUAnCh (max 600 words):
Aldol condensation reaction is an important reaction in valorization of biomass. It uses furfural which is an organic compound obtained from agricultural by-products such as wheat, corncobs. We use light-molecular weight materials such as furfural and acetone in the presence of a catalyst to produce alcohol. The alcohol is then dehydrated to FAc and this is further dehydrated in the presence of the catalyst to generate heavier molecular weight compounds which are used as a precursor in production of biofuel. This reaction usually uses basic catalyst but from technical and environmental consideration, solid materials are preferable. The catalyst I used was called Hydrotalcite (HTC) and it is a solid basic catalyst. HTC is made of cations (Mg2+, Al3+) interlayered with anions (CO32-) and water. In order to activate HTC it needs to be calcined at optimum temperature of 450 °C. The behaviour of this material is not fully understood and is further investigated in my project. One of the unusual behaviours of HTC is its activity dependence on mixing order. We see a significant drop in furfural conversion when we mix HTC with furfural followed by acetone (around 10%) which is much less than when we mix HTC with acetone followed by furfural. We presume this is due to strong interactions of furfural or products of its conversion with active sites of HTC. (Fig 1.)
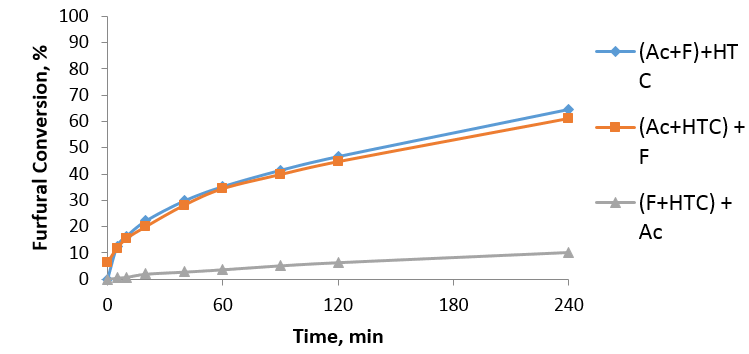
Fig 1
Cannizzaro reaction includes transformation of furfural in the presence of basic catalyst. It involves the formation of furoic acid which, presumably, can interact with basic sites (acid-base interaction) thus blocking active sites for further reaction.
The aim of my research was to find out if there is any possibility of Cannizzaro reaction in the presence of solid base catalyst - HTC (normally this reaction proceeds in the presence of liquid base catalyst). Secondly, to find out the influence of degree of rehydration on properties of HTC in the reaction. Lastly, the extent of deactivation of the HTC during the Cannizzaro reaction and its influence on Aldol Condensation reaction.I have carried out a set of experiments. First, Cannizzaro reaction of furfural with HTC was carried out using different amount of water under different temperatures. The reaction showed that the most conversion was obtained at 50 °C using 12 g of water. This was then used for further reactions. The second set of experiments was to find out the influence of time rehydration on HTC activity. I mixed HTC with water for different amount of time (0, 5, 20, 40, 60, 120 min) before adding furfural. The most yield of alcohol was observed at 0 minutes. This is why we rehydrate HTC in situ (usually it is done outside of reaction mixture). The next step was to perform cannizzaro reaction followed by aldol condensation.
This was to find out the extent of deactivation during cannizzaro reaction and how this influences conversion of furfural in aldol condensation. We found out that after cannizzaro reaction reached the end point (all basic sites blocked) aldol condensation had no furfural conversion. However, upon adding water to the reaction mixture we observe furfural conversion. Nonetheless, with increasing amount of water the conversion of furfural increases. This let us to assume that water creates different sites on HTC which vary in properties. Some of which are active in cannizzaro reaction and some are active in aldol condensation. This is a new research and further investigation of HTC must be done.
Valuable skills and experience gained from working with VUAnCh (max 200 words):
Working in the laboratory at VUAnCh has been a great and at the same challenging experience. I have only worked in the laboratory at university before so coming here I have learnt to work with diverse scientists from different backgrounds.
I had the opportunity to use analytical equipment that I have never used before such as TPD, BET, GC-FID and TGA. I have further developed my research, analytical and laboratory skills as well as the importance on working in a team as part of a research group.

